Medicare Opens New Reimbursement Pathway for MediPines AGM100 Cardiorespiratory Innovation
MediPines is pleased to announce that their AGM100® device has been awarded the prestigious Silver Award for Testing and Diagnostic Products in the 2021 Medical Design Excellence Awards. The Medical Design Excellence Awards are an esteemed award program recognizing cutting-edge achievements in medical product design and celebrating the industry’s foremost manufacturers designing and engineering groundbreaking medical devices that are changing patient care worldwide.
The MediPines AGM100® stood out as the only respiratory medical device recognized in the “testing and diagnostic” product category. This latest honor provides further validation of the revolutionary approach towards non-invasive gas exchange measurements. “The MDEA program is unique in that jurors take every element of the design and engineering process into consideration while also considering benefits to overall healthcare, thus celebrating the companies that are directly facilitating the transformation of patient care delivery…We are proud to applaud the standout companies that exemplify the best in innovation and are driving continued modernization in the highly complex and rapidly evolving health system. Congratulations to each of the 2021 winners.” said Daphne Allen, Editor-in-Chief, MD+DI.
“MediPines is honored by this distinguished MDEA award,” stated Steve Lee, CEO of MediPines. “We are delighted that global industry experts are recognizing the need for this kind of innovation in the respiratory field. The MediPines AGM100’s intuitive and user- friendly design is already having a tremendous impact on patient care, and we are excited that others recognize the excellence that has been achieved.”
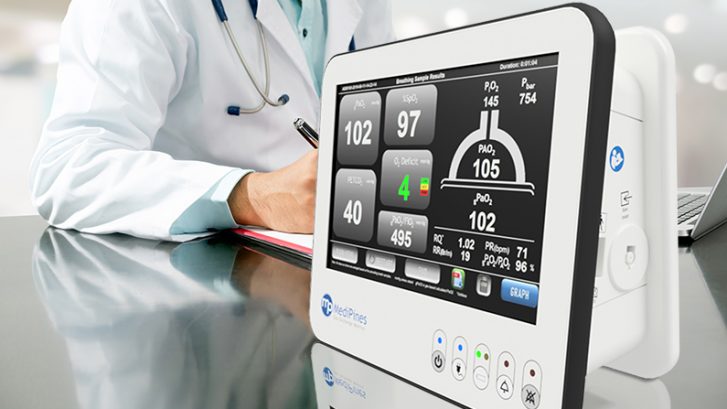
MediPines AGM100®
MediPines AGM100® is the world’s first non-invasive gas exchange analyzer. This advanced respiratory monitoring system was designed to rapidly detect respiratory impairment caused by conditions such as COVID-19, chronic obstructive pulmonary disease (COPD), pneumonia, ARDS, pulmonary edema, and pulmonary embolism. The device is FDA cleared and approved for Health Canada COVID-19 Emergency Use. It provides a comprehensive panel of respiratory measurements including blood oxygen levels, Oxygen Deficit (A-a gradient), P/F ratio, and alveolar oxygen and carbon dioxide levels.
Tags:
May 27, 2021 7:49:45 AM
.png?width=300&height=89&name=MP%20Logo_All-in-One_070820%20(1).png)


